Oxygen State At Room Temperature
Introduction
With oxygen having a much larger electronegativity than hydrogen, most binary d-metal oxides are much more than stable than the corresponding hydrides1,two. Exposed to a gas mixture even at low hydrogen to oxygen ratios of one:10−6, most transition metals form binary oxides instead of ternary hydrido-oxides or oxy-hydrides. Hydroxides practise exist, just tend to decompose into the corresponding oxides likewise3. Still, due to the pocket-sized atomic diameter and the ambivalent grapheme of hydrogen, hydrogen tin can intercalate into oxides and act as a donor or acceptor of electrons4,5,half dozen, beingness the origin of diverse optical and electronic effects with many applications.
The event is particularily stiff in WOiii 7,8 and MoO3 9,10, which accept long been known for their gasochromic properties, and tin can be utilized every bit hydrogen10 and ammonia sensors11. Hydrogen atoms from molecular hydrogen or decomposition of hydrogen containing reducing molecules such equally ammonia intercalate into the oxide and form hydrogen molybdenum bronzes H10Mox 5+Mo1−x 6+Oiii 10. The phase transformation causes only small-scale crystallographic rearrangements (topotactic reduction): hydrogen atoms occupy sites in the van der Waals gaps betwixt double layers of MoOhalf dozen octahedra likewise as intralayer sites on zigzag bondage along the channels12,13. This results in a relatively small increase of the cell volume and slight distortion of the lattice changing the overall crystal symmetry from orthorhombic orthorhombic (phase I) to monoclinic (phases II, IV)12,xiv. Although lattice distortion and hydrogen ordering increase the complication of the system12, the electronic structure may be described using the semiconductor model with hydrogen every bit dopant (Fig. ane). In this model, the MoOiii structure motif (MoO6 octahedrons) remains unchanged and defines the overall electronic construction, but is perturbed past hydrogen atoms every bit new band gap states10. These states change the optical and electrical backdrop: pristine MoOiii consists of Mo6+ forming the conduction bands and O2− forming the valence bands. Pristine MoO3 is semiconducting (and thus transparent) with a band gap of 3.2 eV10. Hydrogen gives its electron to the conduction ring forming protons and "valence band-like Mo5+ states" (see Fig. one: green/gray colored states in the ring gap). As a upshot, a hydrogenated MoO3 film appears blue due to the intervalence-accuse transfer from the newly formed Mo5+ to adjacent Movi+ upon optical excitation. The controversy remains, whether the optical properties are sufficiently described by the intervalence charge transfer theory between Mo5+ and Mohalf dozen+ ions, or by polaron assimilation (small-polaron theory)10, which nonetheless relies on the germination of Mo5+ states. Simultaneously with the color change, the electrical conductivity increases, supporting the doped semiconductor model. At that place is a maximum doping level of hydrogen: exceeding x = 1 in HxMoO3, leads to formation of Mo4+ ions (sketched by the width of the electronic states in Fig. one). However, this situation seems to be unstable. Indeed, at college concentrations water and hydrogen gratis MoOtwo consisting of Mo4+ and O2− states is formed. Every missing oxygen atom is balanced past ii more than electrons in the Mo 4d bands (run across Fig. 1). This film explains the consequence of oxygen defects on the electronic construction of the MoOthree phase as well. The phase transformation is associated with major structural rearrangements and correspondingly high activation barriers. Thus, it is usually but observed at high temperatures or at very high driving forces [see, e.g., ref. xv, and discussion afterwards]. The forerunner of this transformation may be the germination of OH ions equally observed for hydrogen concentrations ten ≫ i by inelastic neutron scattering13. The ambivalent character of hydrogen is like in the related WO3 system: its electronic structure coordinating to the one of MoOiii sketched in Fig. ane depends sensitively on the fact whether H-O-H bridge bonds are formed, or whether hydrogen acts as a delocalized dopant in the semiconductor16.

Left: Simplified crystal structure of MoO3 and hydrogen statuary(due south). Chains of MoO6 octahedrons are fused together by border sharing to grade corrugated layers. The intercalation of hydrogen into MoO3 does not markedly modify the structural motif autonomously from slight increase of the cell volume, lattice distortion and hydrogen ordering12,13,14. Equally already suggested by the vicinity of hydrogens to oxygen, h2o is formed at higher concentrations. In this example, the metal/oxygen ratio is modified leading to MoOii. The changes of the electronic construction by hydrogen intercalation are depicted in the correct console. Hydrogen forms ring gap states (red) and "valence ring-like Mov+ states" (dark-green-rimmed boxes: grey: occupied, white: empty), as depicted by the width of electronic states. The loss of oxygen at higher hydrogen concentrations is depicted past the smaller width of the blue rimmed boxes, simultaneously, more than Mo bands are filled.
In improver to the changes observed during exposure to hydrogen gas, MoO3 is photograph- and electrochromic, and thus used for photo- and electrochromic coatings10. Gasochromism and photo/electrochromism are direct related: optically or electronically excited electrons in the conduction ring can decompose h2o adsorbed at surface and interfaces of MoO3. The produced hydrogen intercalates into the bulk and changes the optical and electronic properties of MoO3 as describe above10.
Thin MoO3 films tin can be quite just prepared by physical11 and chemic vapor deposition17 or via coating-processes using precursor solutions18 or nanoparticle dispersionsnineteen. Thus, MoOiii has been proposed as an anode interfacial layer in organicsixteen,xx,21,22,23,24 and dye-sensitized solar cells24. The low charge injection/extraction barrier at MoO3/organic interfaces is believed to be due to the favorable free energy level alignment between the loftier work function value of MoOthree and the highest occupied molecular orbital of an organic molecule21,22,23. Due to the complex chemistry of this material and the strong impact of external stimuli on the electronic properties, a coherent understanding of the pigsty extraction procedure beyond MoO3 in OPV cells is however defective24. For the application as an electron injection/extraction layer, the formation of hydrogen bronzes by photolysis of hydrogen containing molecules is an undesired effect, considering it diminishes the favorable electronic properties24.
Hydrogen bronzes of MoO3 are metastable states26: they can be further reduced via formation of water to lower oxides and thus the oxygen – metallic ratio depends on the specific experimental conditions. In gasochromic and related applications, the hydrogen concentration is relatively high, and oxygen (water) can exit the oxide resulting in irreversible reduction of the latter to MoO2, and eventually to Mo metallic. The easy reducibility can thus be hypothesized to be the origin of the limited reversibility of such devices. On the other hand, it is assumed to be the source of the remarkable catalytic properties for oxidation reactions of hydrocarbons and alcohols27.
Considering of its evident importance, bulk likewise as surface properties of MoO3 take been carefully studied by surface scientific discipline methods such as photoemission and electron diffraction and bulk methods (e.g., XRD, EXAFS)fifteen,28. Simplified, the outcome is equally follows: at higher temperatures (>700 Yard), the surface of MoOiii is reduced already in vacuum29. Reduction of bulk MoO3 requires high temperature and hydrogen at several mbars, and is proportional to the hydrogen partial pressure level28. The exact decomposition pathway from MoO3 to eventually Mo metallic, in particular the significance of intermediate phases such every bit MofourO7, is still controversially debated. This is due to unprecise experimental conditions (hydrogen purity, in particular the water/oxygen contaminations), and the number of possible reaction steps involved: hydrogen dissociation, hydrogen diffusion, water formation and diffusion, phase nucleation and growth28. Furthermore, the high temperature results are not directly transferable to room temperature, every bit the rate limiting step may change.
In this paper, we apply the XPS-membrane approach30 to clarify the reduction mechanism of MoO3 sparse films at room temperature. X-ray photoelectron spectroscopy (XPS) is an platonic tool to measure the oxidation states of the elements in oxides, run across, due east.g. ref. 31, and has been successfully used to study MoOiii 23,32,33. Different in UHV-XPS-systems, where only post-mortem analysis of ex-situ treated films is possible, this approach allows an operando study of the hydrogenation of MoOthree at relevant hydrogen pressures32. For this, the MoOiii sparse motion picture is deposited on a hydrogen permeable Pd-membrane (run across Fig. ii). The high selectivity for hydrogen diffusion in Pd guarantees an ultrapure quality of hydrogen inbound the MoO3. Furthermore, it is atomic hydrogen interacting with the oxide. The dissociation of hydrogen on oxide surfaces depends on various physico-chemical parameters (among them the oxidation land of the metal). Therefore, the hydrogen flux into the sample of a bare oxide sparse film is difficult to control, in dissimilarity to the membrane arroyo, where it is controlled by adjusting the hydrogen pressure at the dorsum of the membrane. Hydrogen dissociation on Pd is fast equally is the subsequent hydrogen diffusion through the Pd-membrane. Possible rate limiting steps of the reduction of MoO3 are now limited to oxygen diffusion (desorption) and nucleation and growth of new phases. Furthermore, the approach mimics the situation in gasochromic sensors, because also here a Pd layer on top of the thin moving-picture show provides diminutive hydrogen interacting with the layer. The same is true for electrochromic and photochromic devices: water splitting by electrons or photons results in diminutive hydrogens first. Surface science methods are usually limited to UHV-pressures, the membrane approach allows pressures up to 1 bar hydrogen30. In add-on, the membrane provides atomic hydrogen, i.east., hydrogenation of catalytically inactive surfaces such as oxides is possible as well.
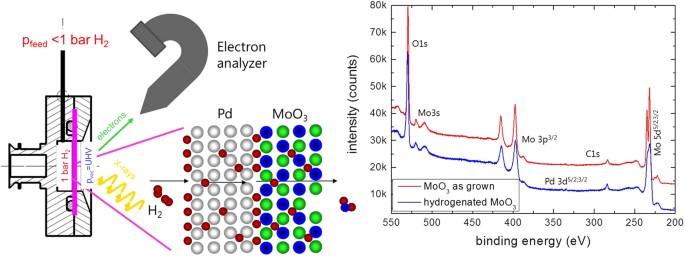
Left panel: membrane approach for XPS measurements. Right panel: XPS-overview spectra of equally deposited MoOiii earlier and after hydrogenation. See besides Methods section.
Results
Figure 2 shows typical XPS-overview spectra of MoO3 deposited on a Pd membrane every bit prepared and after long term hydrogenation. The film thickness of 10 nm is but thick plenty to consider it behaving majority-similar (the interfaces of noble metals to MoO3 are discussed in depth in ref. 21). From the meridian intensities of the oxygen 1 southward and molybdenum 5d peaks, the metallic/oxygen stoichiometry is determined33. In expert understanding with literature, the oxygen content decreases after hydrogenation. However, the oxygen loss is not directly related to the applied hydrogen pressure. This is indicated by the fourth dimension dependence of the process. The improvidence through the Pd-membrane is relatively fast: in previous measurements on hydrogen permeation through the 150 μm thick Pd membrane we observed dynamic equilibrium later on approximately 500 s (run into Methods section, ref. 30) due to the fast hydrogen diffusion in Pd even at room temperature. Nosotros do not expect deceleration by hydrogen diffusion through the 10 nm thin MoOthree movie (the diffusion parameter of H in MoO3 is of the guild of x−11 gtwo/s29,34). However, after an initial hydrogenation at 100 mbar, vaccum was practical. Unexpectedly, a decrease of the oxygen content was measured after hours in vacuum (Fig. three). The rate limiting pace is thus not hydrogen diffusion.
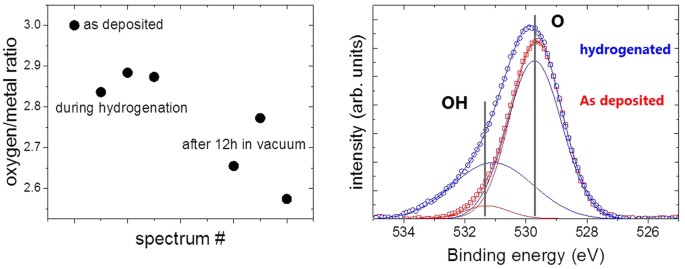
Left panel gives the oxygen/metal ratio during and after hydrogenation at 100 mbar. The correct panel shows details of the oxygen 1 south cadre level spectra equally deposited and subsequently long term exposure to hydrogen (background subtracted). The data is fitted to two lines centered around 529.6 ± 0.2 eV and 531. two ± 0.2 eV, assigned as O and OH, respectively. The intensity ratio between OH and O peaks increases from approximately six% to 50%.
More details are visible afterwards magnifying the spectral regions of the Mo 5d and O 1 south core levels. Figure iii compares the oxygen 1 due south core levels every bit deposited and after long term exposure to hydrogen. The information is fitted to two lines separated by approximately one.6 eV. The value of the main meridian most prominently visble in the equally deposited MoOthree film is in perfect agreement with literature, meet, due east.g. refs 33,35. The second peak may be attributed to oxygen as OH in MoO3 following the discussion of the electronic structure. However, prior to desorption, water formed by the hydration of MoOthree will accumulate at the surface. The existence of the superlative in the as deposited land as well every bit the observed oxygen loss, which proceeds via the desorption of h2o, supports the interpretation of this height as water adsorbed at the surface20. A better probe for the development of the electronic structure during hydrogenation are the Mo 5d states. Figure 4 shows the evolution of the Mo 5d states during hydrogenation at 100 mbar. The shape and positions of the peaks change drastically indicating the change of the oxidation state of Mo from Mo6+ to Mo420,23,32,33,35.
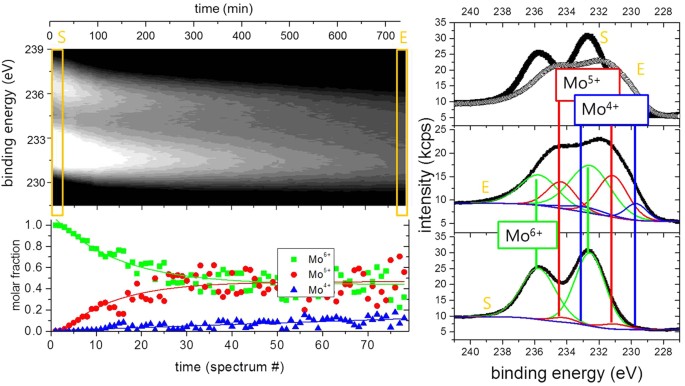
The gray-scale epitome (left height epitome) is a 2d representation of the time evolution. In the right graph, the starting (S) and final (East) spectra are plotted and the applied fits because Mo6+, Mofive+, and Mo4+ 5d 5/2 and 3/2 states are given in the lesser left image. Their intensities are taken every bit measure for the tooth fraction of the respective phases shown with the grey-scale plot.
A plumbing equipment of such complex line shapes is error prone if performed on unmarried spectra alone. Withal, the ability of the membrane arroyo is the possibility to follow the development of the electronic construction operando. Starting situation is divers: Mo6+ states exhibiting two 5d core excitations (5/2 and 3/2). Low hydrogen dose results in boosted peaks assigned to Mo5+. With continued hydrogenation, a shoulder at lowest binding energy develops, which is assigned to Moiv+. To corroborate the formation of Mo4+-states, we compare the reduced MoO3 spectrum with literature data (see Methods Section). All oxidation states display ii peaks20,22,32,33,35. To resolve the spectra, we fit all 6 peaks to predefined fixed binding energies and widths; and the intensities, i.e., only the concentrations are chosen every bit free fitting parameters. This causes a slight scattering of the fitting results, because minor deviations of the exact binding energies every bit a function of the hydrogen concentration are not included. However, the effect is very clear: the growth of the Mo5+ states takes identify at the expense of the Mosix+ states. The respective kinetics is much slower than the diffusion of hydrogen (see discussion above, Methods section). The rate limiting step is thus not the hydrogen supply, nor desorption of water (oxygen, run into Fig. 2), and is therefore assigned to the nucleation and growth of the new HxMoO3−δ phase.
Surprisingly, the Mo4+ land develops independently from this behaviour, and linearly increases over time (Fig. iv). This was likewise confirmed by experiments in which the hydrogen backpressure at the membrane was set to zero, just the evolution of the Mofour+ states and loss of oxygen (encounter Fig. two), i.e., the formation of a molybdenum oxide MoOten with an oxygen content of ten ≪ iii, continued. Equally discussed in the Methods section, the high chemical potential of hydrogen in the MoO3 films is reached on a much faster time scale than the observed development of the Mo4+-states. Thus, the supply of hydrogen is non the rate-limiting footstep. The oxygen content of the new stage associated with Mo4+ is lower than in the hydrogen bronze, and thus oxygen removal is rate-limiting for forming this phase. Due to the racket in the data, just the tendency of decreasing oxygen content is experimentally verified. Locally higher oxygen content at the surface may besides exist realized by reshuffling of oxygen past phase separation into oxygen rich and pure phase as suggested in ref. 33. It is worth noting that the reduced state in one case reached does not reverse back to the original state in vacuum, at least within the measurement time of several days.
Discussion
These results shed light on the kinetic behaviour of the stage formation in non-equilibrium atmospheric condition. Numerous studies investigate the occurrence of intermediate phases during (loftier-temperature) reduction to unravel the decomposition machinery. In particular the 1-footstep reduction mechanism (MoO3 direct to MoO2) is discussed against multiple step mechanisms with additional intermediates being involved (due east.yard. Mo4Oeleven 15). To simplify the give-and-take, we take sketched a qualitative potential energy surface (PES) for the reduction reaction of MoO3 to MoOtwo in Fig. five. The hydrogen statuary is a thermodynamically unstable state26, which will eventually be further reduced because of the globally lower costless energy of MoO2 and water1,15,26. Nonetheless, this iind reduction step is associated with the removal of oxygen by water. For both steps, an free energy barrier has to exist overcome. As the systems has a loftier number n of degree of freedom (reaction coordinates), the PES is a n-dimensional hyper surface, simplified every bit a 2-dimensional surface in Fig. 5. While energy minima can be related to thermodynamic parameters, the barriers and thus the reaction path requires complex calculations of the electronic construction. The potential energy surface displayed here is an educated guess based on empirical figures. We give the enthalpy of formation of the various phases expected to occur to illustrate their location on the energy potential surface shown in Fig. v (data from ref. 26). Every bit a quantitative indicator for the barrier heights, we added the activation energies of reduction at higher temperatures (information from ref. 15). It is worth noting that also the experimentally derived activation energies depend on the pathway.
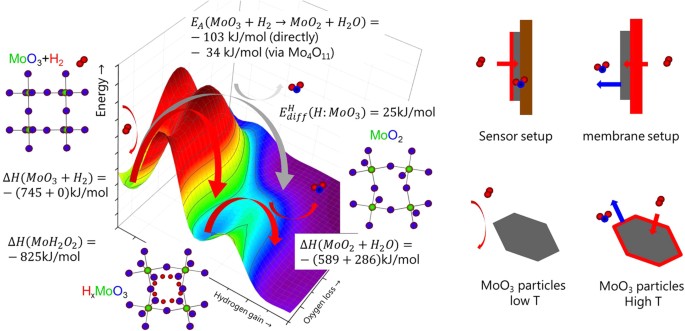
Left: Sketch of the potential energy surface for the reduction of MoO3 to MoOii to depict the possible reaction pathways. The z-centrality is the energy coordinate, while x- and y-axes represent the non further defined reaction coordinates. MoOtwo is near stable with respect to MoO3 and hydrogen, however, hydrogen statuary HxMoOthree is a metastable state. The corresponding enthalpies of formations serve as quantitative estimates for the position on the energy potential surface26. The activation energy of the reactions depends on the path over the potential energy surface. The corresponding experimental activation energies Due east A for the high temperature reduction are indicated15. Hydrogen diffusion is relatively fast in MoO3, which results from the small activation energy for diffusion 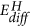 29. The latter is related to the electronic construction on one hand, but the reaction path is too affected by the external weather temperature, hydrogen and h2o partial pressure on the other. These weather depend on the experimental setup as sketched in the right figures: catalysts are commonly made of MoOiii particles, which are basically inert at room temperature, i.e., they practice not dissociate hydrogen nor is desorption of water facilitated (bottom left). At higher temperature, an oxygen scarce, dissociatively active surface is formed with high oxygen mobility (lesser right). To overcome the loftier dissociation at room temperature, one tin coat a MoO3 thin film on an inert substrate with Pd (typical sensor setup, top left). In our membrane approach, the MoOiii thin film is hydrogenated via the Pd substrate, i.e., potentially formed water tin exit the film.
29. The latter is related to the electronic construction on one hand, but the reaction path is too affected by the external weather temperature, hydrogen and h2o partial pressure on the other. These weather depend on the experimental setup as sketched in the right figures: catalysts are commonly made of MoOiii particles, which are basically inert at room temperature, i.e., they practice not dissociate hydrogen nor is desorption of water facilitated (bottom left). At higher temperature, an oxygen scarce, dissociatively active surface is formed with high oxygen mobility (lesser right). To overcome the loftier dissociation at room temperature, one tin coat a MoO3 thin film on an inert substrate with Pd (typical sensor setup, top left). In our membrane approach, the MoOiii thin film is hydrogenated via the Pd substrate, i.e., potentially formed water tin exit the film.
The results at manus as well point that the reaction pathway depends on the charge per unit limiting stride controlled by the external weather condition: in our experiments, the chemical potential of hydrogen supply is very high, and thus the system follows the kinetically favoured path: that is, the intercalation of hydrogen due to the small activation free energy 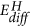 for hydrogen diffusion in MoO3 (encounter Fig. 5, ref. 29) and thus formation of hydrogen bronzes. Withal, the lower reduction state Mo4+ is simultaneously formed, just at a much slower pace (compare E A and
for hydrogen diffusion in MoO3 (encounter Fig. 5, ref. 29) and thus formation of hydrogen bronzes. Withal, the lower reduction state Mo4+ is simultaneously formed, just at a much slower pace (compare E A and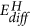 in Fig. v). Oxygen scarce MoO3 surfaces, which can be imagined every bit MoOii inclusions in a MoO3 matrix, tin be formed by heating in UHV without further reductant33. Every bit this takes identify only at high temperatures (big E A ), co-existing phases will exist observed only during low temperature reduction. Furthermore, the multiple steps may not exist sequent steps. The hydrogen statuary is a meta-stable stage, i.e., hydrogen absorption without h2o formation is exothermic26, but further reduction under formation of water is thermodynamically favoured (Fig. 5)1. In addition to the transport of atoms, the kinetics of crystalline phase transformations are limited by long-range order effects. Although the change of the character of hydrogen in the oxide from a delocalized proton to a localized cantlet bridged to oxygen atoms gain without major structural reorientations (compare Fig. ane), the respective changes of the overall electronic structure impact the whole crystal.
in Fig. v). Oxygen scarce MoO3 surfaces, which can be imagined every bit MoOii inclusions in a MoO3 matrix, tin be formed by heating in UHV without further reductant33. Every bit this takes identify only at high temperatures (big E A ), co-existing phases will exist observed only during low temperature reduction. Furthermore, the multiple steps may not exist sequent steps. The hydrogen statuary is a meta-stable stage, i.e., hydrogen absorption without h2o formation is exothermic26, but further reduction under formation of water is thermodynamically favoured (Fig. 5)1. In addition to the transport of atoms, the kinetics of crystalline phase transformations are limited by long-range order effects. Although the change of the character of hydrogen in the oxide from a delocalized proton to a localized cantlet bridged to oxygen atoms gain without major structural reorientations (compare Fig. ane), the respective changes of the overall electronic structure impact the whole crystal.
The local energy minimum of HxMoOiii explains its applied being, but bronzes with high hydrogen concentrations are only formed under loftier chemical hydrogen potentials using atomic hydrogen from chemical reactions (nascent hydrogen), gaseous hydrogen dissociated at Pd overlayers (typical hydrogen sensor setup), or produced electrochemically9,12,26, where water removal is tiresome. The formation of further reduced oxide and water is thermodynamically preferred, explaining the observed irreversibility of the membrane-hydrogenated MoOiii sparse films. Catalysts usually consist of MoOiii particles, which are practically inert at low temperature (Fig. 5). The formation of oxygen deficient surfaces at high temperature enables hydrogen dissociation, and also desorption of h2o is faster at high temperature. In this instance, straight reduction of MoOiii to MoO2 without formation of a bronze is possible. Similar qualitative explanations are valid for the existence of the various other crystalline phases observed during reduction.
The analysis gives guidelines for amend reversibility of devices fabricated of MoOthree. The reduction of Mo should have place without removal of oxygen. This implies the encapsulation of the MoO3 by materials, which exercise non getter oxygen. In hydrogen sensors, this is less a question of the selection of materials (typical setup: substrate/MoOiii/Pdx, Fig. 5), simply of the quality of the thin moving-picture show structure (roughness, coverage)xi. The conditions in organic solar cells are peculiarly hard: here, MoOthree interfaces organic semiconducting small-scale molecules or polymers, possibly even containing residual organic solvents36. Nearly of the chemicals used are reducing and hygroscopic, and thus add an additional thermodynamic driving force for the reduction of MoOthree via water formation, which affects the device performance36. Finally, we notation that the experimental ascertainment of depression temperature reduction to Mo4+ is possible but using the membrane approach guaranteeing high flux of hydrogen atoms into the sample and providing a complimentary surface for water desorption.
To summarize, nosotros demonstrated the operando XPS analysis of the reduction of MoOiii by hydrogen, from which nosotros derived the reaction pathway of the reduction around room temperature. The low temperature results bring together the missing link betwixt reaction mechanisms proposed for bulk materials at loftier temperature hydrogenation and the hydrogen-oxide interactions. Nosotros bear witness that several reaction pathways of the hydrogen reduction take place simultaneously. The preference of one of these pathways can be controlled by the experimental conditions (open/closed setup, exposure to atomic/molecular hydrogen, temperature), but the then subdominant pathway may still occur as side reaction with detrimental effects on the performance of the sought application. E.m., in hydrogen sensors, the reversible intercalation in and out of MoO3 is competing with the unwanted irreversible water formation, which eventually disables the sensor function. This situation is observed in many related materials systems such equally WO3 with a broad range of applications from optically agile sparse films10 and chemic sensors10,xi, hole extraction layers in PV17,37, catalysis27, to solar water splitting38. An important cistron in these applications, which was addressed here for the MoO3 organization, is the transient nature of the various states of matter during hydrogen exposure. The time constant in hydrogen Due west/Mo bronzes is relatively long; however, there are indications of short-lived, just highly mobile and reactive hydrogen states in various other meta-stable systemsiv, to be studied using the membrane approach.
Methods
10 nm thick MoO3 thin films were deposited onto 150 μm thick polycrystalline Pd-membranes by thermal evaporation (0.2 Å/s). In situ XPS surface analysis was performed in a modified VG EscaLab spectrometer with a base of operations pressure below 10−9 mbar. XPS spectra were collected with a SPECS PHOIBOS 100 analyzer using a not-monochromatic Ten-ray source (Al Thou blastoff: 1486.six eV).
The membrane approach is based on a relatively unproblematic setup to overcome the so-chosen pressure gap in photoemission. The core idea of the approach is the membrane device, which is exposed to UHV in the assay chamber on one side and ambience pressure hydrogen on the other. The hydrogen flux from the surface into the vacuum is desorption rather than diffusion limited, leading to the equivalence of ambient pressure conditions at the analysed surface of the membrane (Fig. six). For details of the membrane arroyo we refer to ref. 30. Data evaluation was performed using CasaXPS. For plumbing equipment of the Mo 5d states, we used the post-obit parameters:
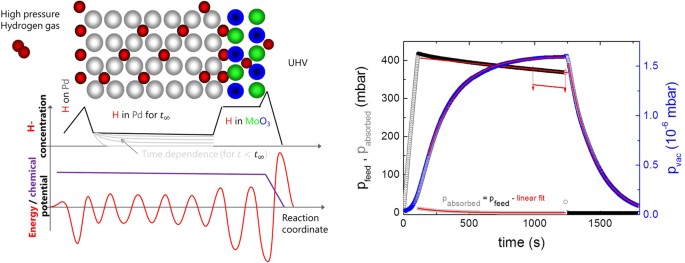
The concentration in a material depends on the hydrogen solubility in the bulk of the membrane setup, and on the chemisorption enthalpy on its surfaces (as sketched by a simplified energy potential diagram). All the same, the chemical potential is balanced by the corresponding entropy terms, so that it is continuous. This is perfectly true for equilibrium weather. The membrane works in quasi-equilibrium. However, because of the low desorption probability of Htwo from MoOiii due to the high activation bulwark, while assimilation of hydrogen on the feed side is facilitated past the small activation energy of hydrogen dissociation on Pd, this quasi-equilibrium is near to equilibrium. In kinetic experiments every bit performed here, quasi-equilibrium is reached within a finite time, which depends on the amount of hydrogen captivated in the majority of the membrane (depicted by greyness lines). The right graph shows the fourth dimension dependence of the pressure at the feed side, which can be converted into the amount of hydrogen entering the membrane, and the force per unit area on the UHV side. A linear decrease of the feed pressure (corresponding to a constant UHV-pressure) is indicative of a constant flux through the membrane, which is reached inside 500 southward. Come across ref. 30 for more details.
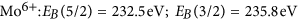
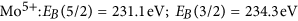
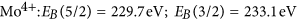
in good agreement with literature information32,33,35,39. The intensity ratio of the 5/2 to 3/two peak was fixed to 5/3, which is also very near to the result obtained from fitting using free parameters of as prepared MoOiii spectra consisting of only half dozen+ states. Figure 7 is a comparison of measurements on membrane-hydrogenated MoO3 and literature information on MoO2 (from ref. 39). Although MoO2 may be described past one Mo4+ and two O2− ions, the XPS shows all three states38.
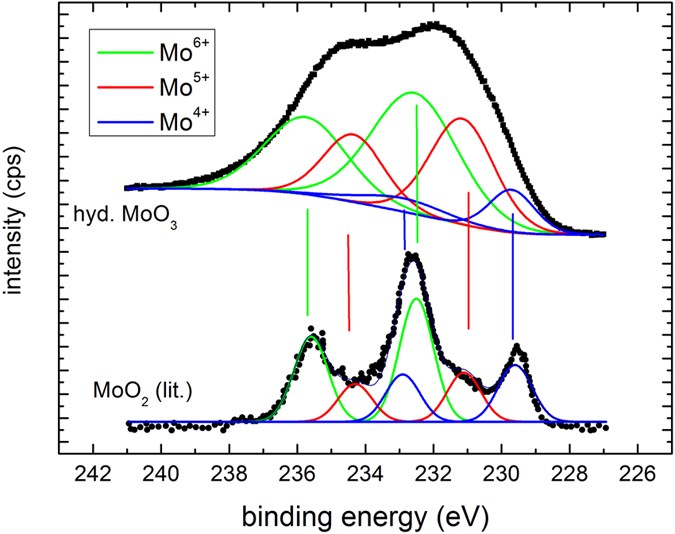
The quality of the fits to the literature information is worse because of digitalization.
Additional Information
How to cite this article: Borgschulte, A. et al. Hydrogen reduction of molybdenum oxide at room temperature. Sci. Rep. vii, 40761; doi: ten.1038/srep40761 (2017).
Publisher's annotation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
Heat of formation of transition metal oxides is of the order of several 100 kJ/mol O, to exist compared to the heats of hydride formation of several 10 kJ/mol H2 and the heat of water formation of 240 kJ/mol; from: Barin, I. Thermochemical Data of Pure Substances threerd edition. (VCH, 1995).
-
Borgschulte, A., Bielmann, K., Züttel, A., Barkhordarian, G., Dornheim, Thou. & Bormann, R. Hydrogen dissociation on oxide covered MgH2 by catalytically active vacancies. Appl. Surf. Sci. 254, 2377–2384 (2008).
-
Holleman, A. F., Wiberg, E. & Wiberg, Northward. Lehrbuch der Anorganischen Chemie 102nd edition. (Gruyter, 2007).
-
Chen, W. P., He, K. F., Wang, Y., Chan, H. L. Due west. & Yan, Z. Highly mobile and reactive state of hydrogen in metal oxide semiconductors at room temperature. Sci. Rep. three, 3149, 10.1038/srep03149 (2013).
-
Van de Walle, C. Chiliad. & Neugebauer, J. Universal alignment of hydrogen levels in semiconductors, insulators and solutions. Nature 423, 626–628 (2003).
-
Van de Walle, C. M. Hydrogen as a cause of doping in ZnO. Phys. Rev. Lett. 85, 1012–1015 (2000).
-
Wöhler, F. Über das Wolfram. Ann. Phys. 78, 345–358 (1824).
-
Berzins, A. R. & Sermon, P. A. Reversible iosthermal sorption of hydrogen by tungsten trioxide in presence of platinum. Nature 303, 506–508 (1983).
-
Glemser, O. & Lutz, Thousand. Über Molybdänblau. Z. Anorg. Allg. Chem. 264, 17–33 (1951).
-
He, T. & Yao, J. Photochromism of molybdenum oxide. J. Photochem. Photobiol. C: Photochem. Rev. four, 125–143 (2003).
-
Mutscha, D., Holzner, One thousand. & Obermeier, East. Sputtered molybdenum oxide sparse films for NH3 detection. Sens. Actuators B Chem. 36, 320–324 (1996).
-
Braida, B., Adams, S. & Canadell, E. Concerning the Structure of Hydrogen Molybdenum Statuary Phase Iii. A Combined Theoretical−Experimental Study. Chem. Mater. 17, 5957–5969 (2005).
-
Dickens, P. G., Birtill, J. J. & Wright, C. J. Elastic and Inelastic Neutron Studies of Hydrogen Molybdenum Bronzes. J. Sol. Sate Chem. 28, 185–193 (1979).
-
Birtill, J. J. & Dickens, P. G. Phase relationships in the system HxMoO3(0 < 10 ~ ii.0). Mater. Res. Bull. 13, 311–316 (1978).
-
Ressler, T., Jentoft, R. E., Wienold, J., Günter, 1000. K. & Timpe, O. In Situ XAS and XRD Studies on the Formation of Mo Suboxides during Reduction of MoO3 . J. Phys. Chem. B 104, 6360–6370 (2000).
-
Vasilopoulou, M. et al. Influence of the oxygen substoichiometry and of the hydrogen incorporation on the electronic band structure of amorphous tungsten oxide films. J. Phys. Chem. C 118, 12632–12641 (2014).
-
Donnadieu, A., Davazoglou, D. & Abdellaoui, A. Construction, optical and electro-optical backdrop of polycrystalline WO3 and MoO3 thin films prepared past chemical vapour degradation. Thin Solid Films 164, 333–338 (1988).
-
Prasada, A. G., Kubinskib, D. J. & Gouma, P. I. Comparing of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection. Sens. Actuators B Chem. 93, 25–30 (2003).
-
Stubhan, T. et al. Loftier shunt resistance in polymer solar cells comprising a MoO3 hole extraction layer processed from nanoparticle intermission. Appl. Phys. Lett. 98, 253308 (2011).
-
Douvas, A. Chiliad. et al. Sol-gel synthesized, depression-temperature processed, reduced molybdenum peroxides for organic optoelectronics applications. J. Mater. Chem. C 2, 6290–6300 (2014).
-
Kröger, M. et al. Role of the deep-lying electronic states of MoO3 in the enhancement of hole-injection in organic sparse films. Appl. Phys. Lett. 95, 123301 (2009).
-
Greiner, M. T. et al. Universal energy-level alignment of molecules on metallic oxides. Nat. Mater. 11, 76–81 (2012).
-
Greiner, M. T., Chai, L., Helander, M. Thou., Tang, Due west. Grand. & Lu, Z. H. Metallic/metal oxide interfaces: how metal contacts affect the work function and band structure of MoO3 . Adv. Funct. Mater. 23, 215–226 (2013).
-
Zhang, H. et al. Photochemical Transformations in Fullerene and Molybdenum Oxide Affect the Stability of Bilayer Organic Solar Cells. Adv. Energy Mater. 5, 1400734 (2015).
-
Kovendhana, One thousand. et al. MoO3 Picture show Counter Electrode for Dye Sensitized Solar Prison cell. AIP Conf. Proc. 1349, 669–670 (2011).
-
Birtill, J. J. & Dickens, P. M. Thermodynamics of Hydrogen bronze Phases HxMoO3 . J. Sol. Land Chem. 29, 367–372 (1979).
-
Grzybowska-Swierkosz, B. Thirty years in selective oxidation on oxides: what have we learned? Elevation. Catal. 11, 23–42 (2000).
-
Słoczynski, J. Kinetics and Mechanism of MoO3 Reduction. Comments on [27]. J. Phys. Chem. B 106, 7718 (2002).
-
Ritter, C., Müller‐Warmuth, W. & Schöllhorn, R. Structure and move of hydrogen in molybdenum bronzes HxMoO3 as studied by nuclear magnetic resonance. J. Chem. Phys. 83, 6130–6138 (1985).
-
Delmelle, R. et al. Closing the pressure gap in Ten-ray photoelectron spectroscopy past membrane hydrogenation. Rev. Sci. Instr. 86, 053104 (2015).
-
Armelao, L. et al. Ion-, photoelectron- and laser-assisted belittling investigation of nano-structured mixed HfO2–SiO2 and ZrO2–SiO2 thin films. Appl. Surf. Sci. 249, 277–294 (2005).
-
Spevack, P. A. & McIntyre, N. S. A Raman and XPS Investigation of Supported Molybdenum Oxide Thin Films. i. Calcination and Reduction Studies. J. Phys. Chem. 97, 11020–11030 (1993).
-
Firment, L. E. & Ferretti, A. Stoichiometric and oxygen scarce MoO3(010) surfaces. Surf. Sci. 129, 155–176 (1983).
-
Adams, S., Ehses, K. H. & Schwitzgebel, K. Proton mobility in single crystals of the hydrogen molybdenum bronzes HxMoO3 . Synth. Met. 43, 3953–3956 (1991).
-
Anwar, M., Hogarth, C. A. & Bulpett, R. Effect of substrate temperature and film thickness on the surface structure of some sparse amorphous films of MoO3 studied by X-ray photoelectron spectroscopy (ESCA). J. Mater. Sci. 24, 3087–3090 (1989).
-
Jenatsch, S. et al. Influence of Molybdenum Oxide Interface Solvent Sensitivity on Accuse Trapping in Bilayer Cyanine Solar Cells. J. Phys. Chem C 118, 17036–17045 (2014).
-
Vasilopoulou, Thou. et al. The Influence of Hydrogenation and Oxygen Vacancies on Molybdenum Oxides Work Function and Gap States for Application in Organic Optoelectronics. J. Am. Chem. Soc. 134, 16178−16187 (2012).
-
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
-
Irfan, So, F. & Gao, Y. Photoemission Spectroscopy Characterization of Attempts to Deposit MoO2 Thin Film. Int. J. Photoenergy 2011, 314702 (2011).
Acknowledgements
This work was partly supported past the UZH-UFSP program LightChEC. Fiscal back up from the Swiss National Science Foundation (grant number 200021_144120) is acknowledged.
Writer information
Authors and Affiliations
Contributions
A.B. and R.D., designed and performed the XPS experiments, South.J. prepared the MoO3 thin films, O.S., R.H. and F.Northward. contributed to the evaluation and scientific discussion of the results. All the authors commented on the manuscript.
Respective author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed nether a Creative Eatables Attribution 4.0 International License. The images or other tertiary party textile in this article are included in the article's Artistic Eatables license, unless indicated otherwise in the credit line; if the material is non included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a re-create of this license, visit http://creativecommons.org/licenses/past/4.0/
Reprints and Permissions
About this article
Cite this article
Borgschulte, A., Sambalova, O., Delmelle, R. et al. Hydrogen reduction of molybdenum oxide at room temperature. Sci Rep 7, 40761 (2017). https://doi.org/10.1038/srep40761
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1038/srep40761
Oxygen State At Room Temperature,
Source: https://www.nature.com/articles/srep40761
Posted by: rosalesvinal1945.blogspot.com


0 Response to "Oxygen State At Room Temperature"
Post a Comment