Lewis Dot Structure For Xef2
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis Construction and Polarity
The XeF4 or Xenon Tetrafluoride is a chemical chemical compound made of Xenon and Fluoride atoms. Information technology is the earth's first binary compound discovered. Information technology is a type of noble gas having the chemical equation of
Xe +2 F2 -> XeF4
The XeF4 has a solid white advent and has a density of 4.040 thousand cm−3 in a solid form. Under ordinary conditions, it appears like a colorless crystalline. It has a sublime temperature of 115.7-caste Celsius or 240.26-degree Fahrenheit. Same as the other Xenon Fluorides, the Xenon Tetrafluoride has an exergonic formation. At normal temperature and pressure, it stays in stable status. Information technology reacts with water instantly and releases molecular oxygen, hydrogen fluoride, and pure xenon gas.
| Name of molecule | Xenon Tetrafluoride (XeF4) |
| No of Valence Electrons in the molecule | 36 |
| Hybridization of XeF4 | sp3d2 hybridization |
| Bond Angles | ninety degrees and 180 degrees |
| Molecular Geometry of XeF4 | Square Planar |
To know more about its physical backdrop and chemical properties, one needs to know its Lewis structure and molecular geometry. Let us observe out the Lewis structure of Xenon tetrafluoride.
For making the Lewis structure, we need to know the valence electrons of XeF4 to brand its construction and know the placement of atoms in the molecule.
XeF4 Valence electrons
In this molecule, we have i atom of Xenon and four atoms of Fluorine. We will calculate the valence electrons of both these atoms to make up one's mind the full number of valence electrons of XeF4.
Valence electrons of Xenon = 8
Valence electrons of Fluorine = 7*4 ( equally at that place are four Fluorine atoms, we will multiply information technology past four)
Total number of valence electrons of Xef4: viii + 7*4
: 8 + 28
: 36
Hence there are a full of 36 valence electrons in XeF4.
XeF4 Lewis Structure
At present that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to depict its Lewis structure. This Lewis dot structure is a pictorial representation of valence electrons around private atoms in a molecule along with the bail it forms.
The bonds in the structure are shown using lines, whereas the electrons not participating in the bond germination are shown as dots. Electrons that practise not form any bail are called nonbonding electrons or lone pairs of electrons.
Hither every bit Xenon is the least electronegative atom, we will place information technology in the centre and all the other fluorine atoms effectually it similar this:

Now that we take placed all the atoms let's bear witness bonds between each Fluorine and Xenon atom. Each bond in the molecule takes up ii electrons, and as there are four single bonds in this molecule, eight electrons out of 36 are used up.
Commencement placing the rest of the valence electrons around the atoms. Each fluorine atom will have 6 valence electrons around information technology, every bit one electron was used to make the bond.
You might discover that we have already placed 24 electrons out of 28 around the fluorine atoms. The remaining nonbonding electrons or lone pairs of electrons volition be placed on Xenon as it is an exception to the octet dominion.
Place these two pairs of nonbonding electrons on Xenon, and at present you take a Lewis structure where there are two alone pairs of electrons on Xenon and six nonbonding electrons on each Fluorine atom.
XeF4 Hybridization
The central Xenon atom'south orbitals are hybridized, which results in the formation of new hybridized orbitals. Xenon has half-dozen electrons in its 5p orbitals and two electrons in 5s orbitals. There are no electrons in d-orbitals and f-orbitals in the ground country of Xenon. But when this atom is in an excited state, ii electrons in the p-orbitals motility to d-orbitals; as a issue, there are four unpaired electrons in total. Out of which, two are in p-orbitals, and the other two unpaired electrons are in d-orbitals. These hybridized orbitals lead to sp3d2 hybridization in XeF4.
XeF4 Molecular Geometry
It is easier to understand the molecular geometry of a given molecule once nosotros know its Lewis structure. As Xenon has two alone pairs of electrons, it will have upward a structure that helps these lone pairs avoid the repulsion forces. To keep these repulsions at a minimum, the lone pairs will be in a perpendicular plane. And every bit there are four fluorine atoms, the molecule will have an arrangement such that its molecular geometry is foursquare planar. XeF4 has an electronic geometry of octahedral, making the molecular geometry of Xenon Tetrafluoride square planar.
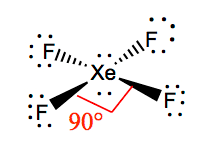
XeF4 Bail angles
The bond angles of F-Xe-F are ninety degrees, and lone pairs accept angles of 180 degrees. The Fluorine atoms are located at 90 degrees to each other, resulting in the symmetric distribution of the electrons in the molecule's airplane. These bond angles contribute to the formation of foursquare planar molecular geometry.
XeF4 Polarity – Is XeF4 Polar or Nonpolar?
Although the bonds between Xenon and Fluorine atoms are polar, XeF4 is a nonpolar molecule. Wondering how? All the Xe-F bonds are in opposition with each other mutually, making the sum of dipole moment naught. As there are four electrons on the Xenon atom, which are localized as nonbonding pairs of electrons. As the overall arrangement of the atoms and electrons in the molecule is such that the vector sum of the dipoles is zero, XeF4 is a nonpolar molecule.
Final Remarks
Xenon Tetrafluoride is one of those molecules that is relatively like shooting fish in a barrel to understand. Its Lewis structure is one of the least complicated structures, as all the Fluorine atoms are bundled in the symmetric pattern. The alone pairs in the molecule are located in a perpendicular plane in an octahedral shape to go on their repulsive forces at a minimum.
To summarize this weblog post, we can say that XeF4 has 36 valence electrons. It has two alone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron geometry and square planar molecular geometry. XeF4 is a nonpolar molecule and has sp3d2 hybridization.
At the Geometry of Molecules, we similar knowing what you lot retrieve. So let us know your thoughts on this molecule in the comments below.
Lewis Dot Structure For Xef2,
Source: https://geometryofmolecules.com/xef4-xenon-tetrafluoride-molecular-geometry-lewis-structure-polarity/
Posted by: rosalesvinal1945.blogspot.com


0 Response to "Lewis Dot Structure For Xef2"
Post a Comment